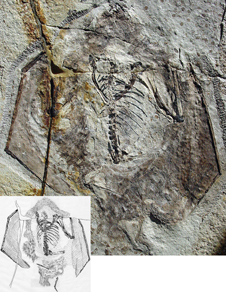
The anurognathids are a weird bunch no doubt. A very short tail for a non-pterodactyloid pterosaur, multiple layers of actinofibrils (Kellner et al. 2009), short skull / large orbit (Bennett 2007) and a hairy body (Wang et al. 2002) to name a few of their more obvious structures. I could also mention the three phalanges of the fourth digit but this interpretation appears to have been challenged lately.
During our recent visit to Beijing a couple of my colleagues were fortunate to get to spend a week pouring over the superb specimen of Jeholopterus ningchengensis (IVPP V12705) for some research on membrane composition and structure. The recurring point that cropped up in a number of conversations was that however well preserved the specimen is, anurognathids are just such strange animals that it is potentially dangerous to regard them as being typical for anything other than anurognathids i.e. can we really apply what we know about anurognathid membrane structure / composition to other pterosaurs?
This will certainly be an interesting question the group will have to consider in the immediate future but in this post I wanted to quickly draw attention to a part of the wing that often gets overlooked…..the hair-like structures along the trailing edge. Kellner et al. 2009 published a small photograph of this but I’m not aware of much in the literature that covers its potential functions (although I’m without a copy of “Deep Time” so apologies if it has been covered before).
Combs along the trailing edge of the wing (or turbine when we enter the realm of man-made machines) are known to reduce the noise produced by the animal during flight by collapsing the vortex shed off the wing. There is quite a bit of literature on this subject but its function in biological flight could warrant further attention. Owls are certainly the most famous example where these structures are used to reduce the noise produced by the wings at frequencies above 2 kHz – i.e. within the hearing range used by both the owl and its prey (Bachmann et al. 2007). As a result owls are masters of silent flight which allows them to approach their prey to a much greater degree than would otherwise be possible.
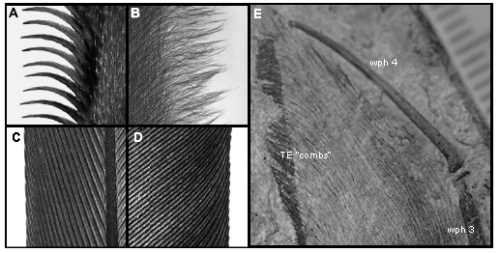
A) LE of owl primary 10; B) TE of owl primary 10; C) LE of pigeon primary 10; D) TE of pigeon primary 10; E) TE combs of Jeholopterus. Adapted from Bachmann et al. 2007. LE = leading edge; TE = trailing edge.
The examples above show just how similar the trailing edge structures of the wing in Jeholopterus (E) are when compared to those of an owl (A, B) and the obvious differences they have with those of a noisy flier, in this case a pigeon (C, D). While I am in no way trying to compare the functionality of a feather with that of the pterosaur membrane, the presence of trailing edge structures would almost certainly function in a similar way to other noise reduction structures.
Jeholopterus shares a couple of similarities with that of the barn owl: they both use a slow flight while hunting and both were active during times of darkness or at least periods of low light, but there the similarities end. Jeholopterus was almost certainly an insectivore rather than hunting small vertebrates and so it is not immediately apparent how effective a noise reducing structure like this would have been (hearing range of insects anyone?). So could Jeholopterus have used its trailing edge structures to break down the vortex shed off its wing? If so it would have flapped and glided over the darkening Mesozoic landscape using a combination of slow flight speed, high maneouverability and specialised fibres to reduce the noise frequency of the wings, snapping up insects as it went. Certainly some food for thought regardless.
References:
Bachmann, T., Klän, S., Baumgartner, W., Klaas, M., Schröder, W. and Wagner, H. 2007. Morphometric characterisation of wing feathers of the barn owl Tyto alba pratincola and the pigeon Columba livia. Frontiers in Zoology 4, doi:10.1186/1742-9994-4-23.
Bennett, S.C. 2007. A second specimen of the pterosaur Anurognathus ammoni. Paläontologische Zeitschrift 81: 376-398.
Kellner, A.W.A., Wang, X., Tischlinger, H., Campos, D.A., Hone, D.W.E. and Meng, X. 2009. The soft tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the structure of the pterosaur wing membrane. Proc. Roy. Soc. B. doi:10.1098/rspb.2009.0846.
Wang, X., Zhou, Z., Zhang, F., and Xu, X. 2002. A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and ‘hairs’ from Inner Mongolia, northeast China. Chinese Science Bulletin 47: 226 – 232.